Biochemical Oxygen Demand (BOD) is a key indicator of the health of a water body. It reflects the amount of oxygen required by microorganisms to decompose organic matter in water. This parameter is widely used in environmental science, wastewater management, and water pollution control to assess the level of organic pollution in natural and artificial water systems.
Whether you’re preparing for competitive exams like UPSC, TNPSC, or learning about environmental studies, understanding BOD is essential.
Table of Contents
What is Biochemical Oxygen Demand (BOD)?
Biochemical Oxygen Demand (BOD) is the amount of dissolved oxygen required by aerobic microorganisms to decompose organic matter present in a given water sample over a specific time period — usually 5 days at 20°C.
It is a key indicator of the organic pollution level in water and helps assess water quality in rivers, lakes, and treatment plants.
Definition of BOD
Biochemical Oxygen Demand (BOD) is defined as the amount of dissolved oxygen consumed by microorganisms during the biological decomposition of organic matter under aerobic conditions in a specific period.
Key Points:
- Measured in mg/L (milligrams per liter) or ppm (parts per million)
- Typically assessed over 5 days at 20°C (referred to as BOD₅).
- Indicates organic pollution levels.
- Higher BOD = more organic matter = poor water quality.
Why is BOD Important?
BOD serves as a crucial parameter in environmental monitoring and wastewater treatment. Here’s why it’s important:
- Indicator of Organic Pollution: High BOD levels indicate significant organic matter in water, often from sewage, agricultural runoff, or industrial discharges, which can deplete oxygen levels and harm aquatic life.
- Aquatic Ecosystem Health: Low dissolved oxygen levels due to high BOD can lead to hypoxic conditions, causing fish kills and disrupting ecosystems.
- Wastewater Treatment Efficiency: BOD is used to evaluate the performance of wastewater treatment plants by measuring the reduction in organic load before and after treatment.
- Regulatory Compliance: Environmental agencies, such as the U.S. Environmental Protection Agency (EPA) or the European Environment Agency, set BOD limits for wastewater discharges to protect water bodies.
- Water Resource Management: BOD data informs decisions on water treatment, pollution control, and sustainable water use.
Factors Affecting BOD
Several factors influence BOD measurements and the rate of oxygen consumption:
- Organic Matter Content: Higher concentrations of biodegradable organic matter (e.g., sugars, proteins) increase BOD.
- Microbial Population: The presence and diversity of aerobic microorganisms affect the rate of organic decomposition.
- Temperature: The standard test uses 20°C, but higher temperatures accelerate microbial activity, increasing oxygen demand.
- pH: Optimal microbial activity occurs at pH 6.5–7.5. Extreme pH values can inhibit microorganisms.
- Toxicity: Toxic substances (e.g., heavy metals, pesticides) in the sample can suppress microbial activity, reducing BOD.
- Dissolved Oxygen Availability: If DO is depleted during the test, results may underestimate the true BOD.
- Nitrification: If nitrifying bacteria are present, NBOD can contribute significantly, requiring inhibitors (e.g., allylthiourea) to measure CBOD alone.
BOD vs. COD: What’s the Difference?
While both BOD and Chemical Oxygen Demand (COD) measure organic pollution, they differ significantly:
| Feature | Biochemical Oxygen Demand (BOD) | Chemical Oxygen Demand (COD) |
|---|---|---|
| Basis | Biological decomposition of biodegradable organic matter | Chemical oxidation of both biodegradable and non-biodegradable organic matter |
| Timeframe | Typically 5 days (BOD5) | 2-3 hours |
| Reagents | Microorganisms (biological process) | Strong chemical oxidants (e.g., potassium dichromate) |
| Measures | Only biodegradable organic matter | Almost all oxidizable organic and inorganic matter |
| Application | Wastewater treatment efficiency, water quality assessment | Rapid assessment of pollution, industrial wastewater |
BOD in Environmental Contexts
Impact on Aquatic Life
High BOD leads to a depletion of dissolved oxygen in water bodies. This creates anaerobic conditions that are detrimental to most aquatic organisms, including fish, macroinvertebrates, and beneficial bacteria. This can result in:
- Fish kills
- Loss of biodiversity
- Odor problems (due to anaerobic decomposition)
Wastewater Treatment
Wastewater treatment plants are designed to reduce the BOD of influent water before discharge. The primary goal of biological treatment processes (e.g., activated sludge, trickling filters) is to allow microorganisms to consume the organic matter, thereby lowering the BOD.
Effluent BOD: Low (e.g., <30 mg/L for treated effluent)
Influent BOD: High (e.g., 200-400 mg/L for municipal sewage)
BOD vs. Other Water Quality Parameters
| Parameter | Description | Measures | Advantages | Limitations |
|---|---|---|---|---|
| BOD | Oxygen consumed by microorganisms over 5 days | Biodegradable organic matter | Specific to microbial activity, reflects ecological impact | Time-intensive, misses non-biodegradable pollutants |
| COD | Oxygen required to chemically oxidize organic/inorganic matter | Total oxidizable matter | Faster (2–3 hours), includes non-biodegradable matter | Less specific, uses hazardous chemicals |
| TOC | Total carbon content in organic compounds | Organic carbon | Quick, no chemicals needed | Does not directly measure oxygen demand |
| DO | Dissolved oxygen in water | Immediate oxygen availability | Real-time, direct measurement | Snapshot, not a pollution indicator |
Typical BOD Values
BOD levels vary by water type:
- Pristine rivers/lakes: 1–2 mg/L (low organic matter).
- Moderately polluted rivers: 5–10 mg/L.
- Untreated sewage: 200–600 mg/L.
- Treated wastewater effluent: 10–30 mg/L (post-treatment).
- Industrial effluents (e.g., food processing): 1,000–10,000 mg/L.
High BOD (>10 mg/L in natural waters) can lead to oxygen depletion, causing anaerobic conditions and foul odors.
✅ Explore the history and impact of No-Confidence Motions in Lok Sabha – Timeline, Procedure & Key Events, essential for Indian Polity and UPSC exam preparation.
How is BOD Measured? The BOD5 Test
The most common method for determining BOD is the BOD5 test. This involves:
- Sample Collection: A water sample is collected and properly preserved.
- Dilution: The sample is diluted with aerated distilled water containing essential nutrients and buffer to ensure optimal microbial activity.
- Initial DO Measurement (Day 0): The initial dissolved oxygen (DO) content of the diluted sample is measured.
- Incubation: The diluted sample is incubated in the dark at a standard temperature, typically 20°C, for 5 days. Incubation in the dark prevents photosynthetic oxygen production by algae, which could skew results.
- Final DO Measurement (Day 5): After 5 days, the DO content of the incubated sample is measured again.
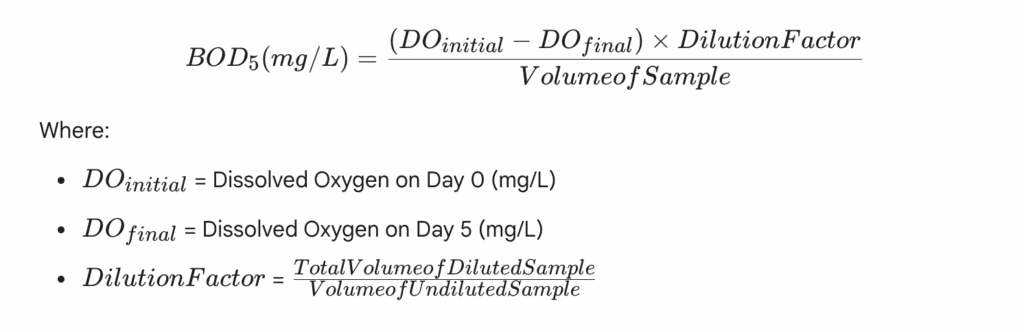
- Calculation: The BOD5 is calculated based on the difference between the initial and final DO concentrations, taking into account the dilution factor.
Formula for BOD5 Calculation:
Key Takeaways for Exam Aspirants
- BOD is a measure of biodegradable organic pollution.
- The BOD5 test is the standard, involving 5 days of incubation at 20°C.
- High BOD indicates poor water quality and oxygen depletion risk.
- BOD is crucial for assessing wastewater treatment efficiency.
- Understand the difference between BOD and COD.
- Remember the formula for BOD5 calculation.
By thoroughly understanding these concepts, you’ll be well-prepared to tackle any question related to Biochemical Oxygen Demand in your upcoming examinations. Good luck with your studies!
👉 For in-depth academic insights, explore this detailed article on Biochemical Oxygen Demand (BOD) at ScienceDirect.
Frequently Asked Questions (FAQs) about Biochemical Oxygen Demand (BOD)
What is Biochemical Oxygen Demand (BOD)?
BOD, or Biochemical Oxygen Demand, measures the amount of dissolved oxygen consumed by aerobic microorganisms to break down organic matter in a water sample over a specific period, typically 5 days at 20°C. It’s a crucial indicator of water quality and organic pollution.
What is the difference between BOD and COD?
1. BOD measures only biodegradable organic matter using microorganisms over 5 days.
2. COD measures both biodegradable and non-biodegradable matter using chemical oxidants in just 2–3 hours.
COD provides a faster, broader measure of total oxidizable substances, while BOD is specific to biological activity and its impact on aquatic life.How is BOD measured, specifically the BOD5 test?
The most common method is the BOD5 test. It involves:
1. Sample collection and preservation.
2. Dilution of the sample with aerated water and nutrients.
3. Measuring initial dissolved oxygen (DO) (Day 0).
4. Incubation of the diluted sample in the dark at 20°C for 5 days.
5. Measuring final DO (Day 5).
6. Calculating BOD5 based on the DO difference and dilution factor.What do high BOD values indicate about water quality?
A high BOD value (e.g., >10 mg/L in natural waters) signifies poor water quality and a significant amount of organic pollution. This often leads to oxygen depletion (hypoxia or anoxia), creating anaerobic conditions, which can cause fish kills, loss of biodiversity, and foul odors due to anaerobic decomposition.
What are typical BOD values in different water sources?
1. Pristine water: 1–2 mg/L
2. Moderately polluted: 5–10 mg/L
3. Untreated sewage: 200–600 mg/L
4. Treated wastewater: 10–30 mg/L
5. Industrial effluents: 1,000+ mg/LWhich factors affect BOD readings?
Several factors influence BOD results:
* Organic matter content: Higher concentrations increase BOD.
* Microbial population: Presence and diversity affect decomposition rate.
* Temperature: The standard is 20°C, but higher temperatures accelerate microbial activity.
* pH: Optimal activity is at pH 6.5–7.5; extremes inhibit microbes.
* Toxicity: Toxic substances can suppress microbial activity, leading to underestimated BOD.
* Dissolved Oxygen availability: Insufficient DO can lead to underestimated BOD.
* Nitrification: Nitrogenous BOD (NBOD) can contribute; inhibitors are used to isolate CBOD.Why is the BOD test conducted in the dark?
To prevent photosynthesis by algae, which produces oxygen. Light exposure could lead to inaccurate results by artificially raising oxygen levels during incubation.
Where is BOD testing commonly used?
In wastewater treatment plants, river water testing, environmental impact assessments, and during pollution control measures.
How is BOD used in wastewater treatment?
BOD is a primary metric for wastewater treatment plants. They aim to significantly reduce the BOD of incoming (influent) water (e.g., from 200-600 mg/L for municipal sewage) to meet discharge limits for treated effluent (e.g., <30 mg/L). Biological treatment processes specifically target the microbial consumption of organic matter to lower BOD.
Why is BOD important for water quality assessment?
BOD is vital because it reveals the level of biodegradable organic pollution in water. High BOD levels indicate excessive organic waste, which leads to oxygen depletion, harming aquatic life like fish and disrupting aquatic ecosystems. It’s also used to evaluate wastewater treatment efficiency and ensure regulatory compliance.
